Listen to the article
FDA Targets 18 Websites Selling Counterfeit Botox Products Amid Safety Concerns
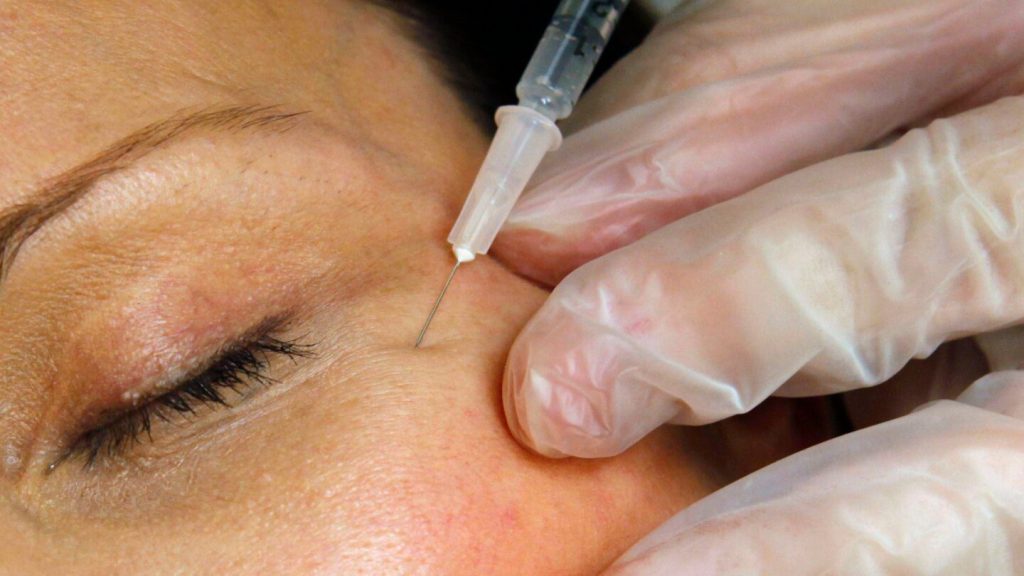
U.S. health regulators have taken action against 18 websites selling counterfeit or unapproved versions of Botox and similar injectable wrinkle treatments, following reports of injuries and toxic side effects associated with these products.
The Food and Drug Administration (FDA) issued warning letters to primarily cosmetic websites offering unauthorized or mislabeled versions of botulinum toxin-based products that have not received regulatory approval. The agency emphasized that these counterfeit products pose significant health risks to consumers.
Botox, which first received FDA approval in 1989 through manufacturer Allergan (now part of AbbVie), contains a diluted, purified form of botulinum toxin—one of the world’s most potent toxic substances. The treatment works by temporarily blocking nerve signals, causing muscle relaxation that reduces the appearance of wrinkles.
While widely recognized for its cosmetic applications, Botox has also secured FDA approval for numerous medical conditions, including chronic migraines, excessive sweating, overactive bladder, and various muscle spasm disorders. This versatility has helped fuel the product’s popularity, with global Botox sales exceeding $4.8 billion annually, according to recent market reports.
Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, expressed concern over the rising trend of illicit cosmetic injectables. “The proliferation of counterfeit injectables represents a serious public health threat,” Marks said in a statement. “These products bypass crucial safety protocols and manufacturing standards that protect patient safety.”
All FDA-approved botulinum toxin products carry the agency’s most serious warning—a black box label alerting healthcare providers and patients to potential life-threatening side effects. In rare but severe cases, the toxin can spread beyond the injection site to other body parts, potentially paralyzing or weakening muscles essential for breathing and swallowing.
Symptoms of botulism poisoning include difficulty swallowing or breathing, slurred speech, and muscle weakness, which can appear several hours after an injection. The FDA urges anyone experiencing these symptoms to “seek immediate medical care,” as prompt treatment is critical in managing toxin-related complications.
The regulatory action comes amid a booming market for cosmetic injectables, which has seen explosive growth during the post-pandemic period. Industry analysts estimate the global facial injectable market will reach $25 billion by 2025, creating incentives for counterfeit products to enter the supply chain.
Health authorities have documented an increasing number of adverse events tied to unauthorized injectables, including infections, permanent tissue damage, and severe allergic reactions. Last year alone, U.S. Customs and Border Protection seized over 14,800 units of counterfeit Botox products valued at more than $1.2 million.
The FDA emphasized that patients should only receive these treatments from licensed and properly trained healthcare professionals. Legitimate providers obtain Botox and similar products through authorized distribution channels, ensuring product authenticity and proper storage conditions.
Industry experts recommend that consumers verify their provider’s credentials and ask to see the product packaging before receiving treatment. Authentic Botox vials feature holographic seals, lot numbers, and expiration dates that can be verified through the manufacturer.
The FDA’s enforcement action represents part of a broader effort to combat counterfeit pharmaceutical products, which the World Health Organization estimates generate over $200 billion annually in illegal revenue worldwide.
Consumers can report suspected counterfeit products or adverse events through the FDA’s MedWatch program, which helps regulators identify and track potential safety issues in the marketplace.
Fact Checker
Verify the accuracy of this article using The Disinformation Commission analysis and real-time sources.



8 Comments
This is a concerning issue. Counterfeit Botox could be made with unknown and potentially toxic substances, putting users at serious risk. I’m glad the FDA is taking action to shut down these illegal websites and warn the public.
The FDA is right to be vigilant about unapproved Botox. While cosmetic procedures can be tempting, using untested products carries serious health risks. Consumers should always consult licensed medical professionals for any injectable treatments.
Agreed. Safety has to come first with medical procedures, even cosmetic ones. Good to see the FDA enforcing regulations to protect people from potentially harmful counterfeit products.
The FDA is right to crack down on websites selling unapproved Botox. Consumers should be wary of any online offers for injectable treatments, as the safety and efficacy of these products cannot be guaranteed. It’s best to consult licensed professionals for any cosmetic procedures.
Concerning to hear about counterfeit Botox products causing safety issues. It’s critical that consumers only use FDA-approved treatments from legitimate sources to avoid potential health risks. Proper oversight and enforcement are important to protect public wellbeing.
Absolutely. Counterfeit medical products can be extremely dangerous. I’m glad the FDA is taking action to crack down on these illegal websites selling unapproved Botox.
While the cosmetic appeal of Botox is understandable, using unapproved versions can have dangerous consequences. It’s crucial that people only seek out legitimate, FDA-regulated treatments from qualified medical providers to ensure their safety.
Completely agree. The risks of counterfeit Botox far outweigh any potential cosmetic benefits. Proper oversight and regulation are essential to protect public health.